Element Lead (Pb), Group 14, Atomic Number 82, p-block, Mass 207.2. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. … Melting point The temperature at which the solid-liquid phase change occurs. Boiling point The temperature at which the liquid-gas phase change occurs.
Everything You Need to Know About: Aluminum
The melting point of lead (Pb) is 327.46 degrees Celsius or 621.43 degrees Fahrenheit.

Source Image: blog.thepipingmart.com
Download Image
Lead is a chemical element; it has symbol Pb (from Latin plumbum) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air.

Source Image: pipedrive.com
Download Image
Melting point of lead at 100atm Thermal Properties of Lead Lead – Melting Point and Boiling Point. Melting point of Lead is 327.5°C. Boiling point of Lead is 1740°C. Note that, these points are associated with the standard atmospheric pressure. Boiling Point. In general, boiling is a phase change of a substance from the liquid to the gas phase.

Source Image: engineeringtoolbox.com
Download Image
What Is The Melting Point Of Lead
Thermal Properties of Lead Lead – Melting Point and Boiling Point. Melting point of Lead is 327.5°C. Boiling point of Lead is 1740°C. Note that, these points are associated with the standard atmospheric pressure. Boiling Point. In general, boiling is a phase change of a substance from the liquid to the gas phase. The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. … Lead: 11.342: 600.61: 2,022 Bismuth: 9.807: 544.7: 1,837 Notes
Matal Alloys – Melting Points
Melting point of Lead is 327.5°C. Note that, these points are associated with the standard atmospheric pressure. In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. lead is a highly malleable metal that has a low melting pointTama o Mali – Brainly.ph

Source Image: brainly.ph
Download Image
Solved] Statement (I): Melting point of alloy containing 62% ti Melting point of Lead is 327.5°C. Note that, these points are associated with the standard atmospheric pressure. In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs.
![Solved] Statement (I): Melting point of alloy containing 62% ti](https://storage.googleapis.com/tb-img/production/20/06/F5_S.C_Madhu_19.06.20_D5.png)
Source Image: testbook.com
Download Image
Everything You Need to Know About: Aluminum Element Lead (Pb), Group 14, Atomic Number 82, p-block, Mass 207.2. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. … Melting point The temperature at which the solid-liquid phase change occurs. Boiling point The temperature at which the liquid-gas phase change occurs.

Source Image: blog.boydmetals.com
Download Image
Melting point of lead at 100atm Lead is a chemical element; it has symbol Pb (from Latin plumbum) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air.
Source Image: physicsforums.com
Download Image
What is the Melting Point of Stainless Steel? Fact file. Atomic number 82; atomic mass 207.2; melting point 327°C; boiling point 1749°C; density 11.4 g cm -3 . Lead is a member of group 14 of the periodic table. It has oxidation states +2 (PbCl 2, Pb 2+ ), and +4 (PbCl 4, PbO 2, which is a powerful oxidising agent.) Bookmark.

Source Image: marlinwire.com
Download Image
Share it! Science : Exploring Melting Point: Turkey Timer STEM Thermal Properties of Lead Lead – Melting Point and Boiling Point. Melting point of Lead is 327.5°C. Boiling point of Lead is 1740°C. Note that, these points are associated with the standard atmospheric pressure. Boiling Point. In general, boiling is a phase change of a substance from the liquid to the gas phase.

Source Image: shareitscience.com
Download Image
Lead (Pb). Diagram of the nuclear composition and electron configuration of an atom of lead-208 (atomic number: 82), the most common isotope of this e Stock Photo – Alamy The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. … Lead: 11.342: 600.61: 2,022 Bismuth: 9.807: 544.7: 1,837 Notes
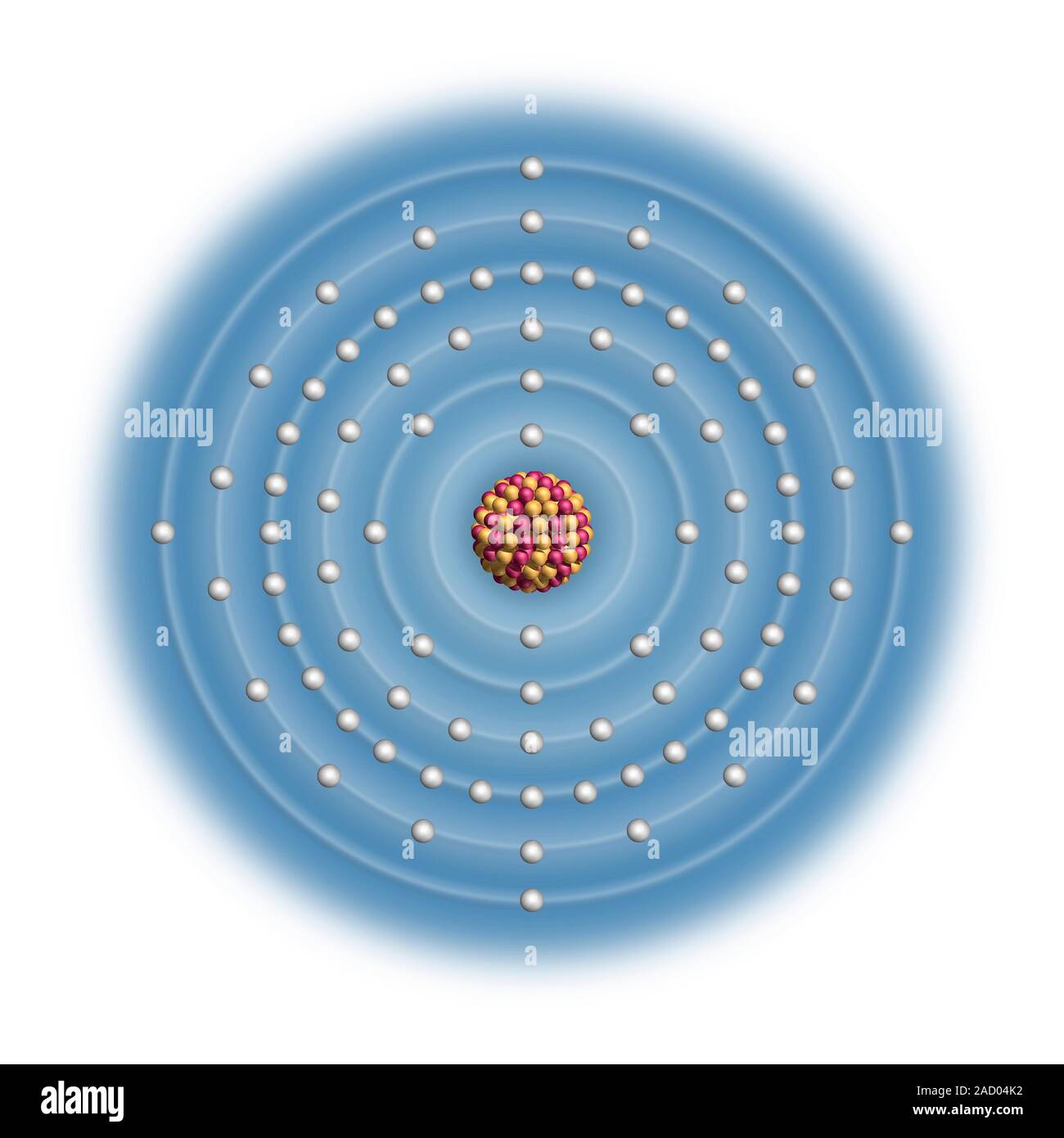
Source Image: alamy.com
Download Image
Solved] Statement (I): Melting point of alloy containing 62% ti
Lead (Pb). Diagram of the nuclear composition and electron configuration of an atom of lead-208 (atomic number: 82), the most common isotope of this e Stock Photo – Alamy The melting point of lead (Pb) is 327.46 degrees Celsius or 621.43 degrees Fahrenheit.
Melting point of lead at 100atm Share it! Science : Exploring Melting Point: Turkey Timer STEM Fact file. Atomic number 82; atomic mass 207.2; melting point 327°C; boiling point 1749°C; density 11.4 g cm -3 . Lead is a member of group 14 of the periodic table. It has oxidation states +2 (PbCl 2, Pb 2+ ), and +4 (PbCl 4, PbO 2, which is a powerful oxidising agent.) Bookmark.