The oxygen atom has a 2− charge as an ion. To balance the positive and negative charges, we look to the least common multiple—6: two iron 3+ ions will give 6+, while three 2− oxygen ions will give 6−, thereby balancing the overall positive and negative charges. Thus, the formula for this ionic compound is Fe 2 O 3.
393 Calcium Ions Royalty-Free Photos and Stock Images | Shutterstock
Roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron(II) has a 2+ charge; iron(III) a 3+ charge. Anions 1-acetate C 2 H 3 O 2-cyanide CN-amide NH 2-cyanate OCN-hydrogen carbonate fluoride F-(bicarbonate) HCO 3-hydride H-

Source Image: yifengtuo.en.made-in-china.com
Download Image
The number in the name of the compound close compound A substance formed by the chemical union of two or more elements. shows the charge of the metal ions in that compound. For example, copper(II

Source Image: brewbitz.com
Download Image
What is TDS in Water & Why Should You Measure It? – Fresh Water Systems Nov 21, 2023Yes. Calcium has a positive 2 charge when it is in its ion form. This would mean that calcium has lost its 2 valence electrons and has 2 more protons than electrons. Calcium Ion

Source Image: studypool.com
Download Image
If Calcium Ions Each Of Which Has A Charge Of
Nov 21, 2023Yes. Calcium has a positive 2 charge when it is in its ion form. This would mean that calcium has lost its 2 valence electrons and has 2 more protons than electrons. Calcium Ion Sep 21, 2023bajajsakshi1995 Final answer: If calcium ions with a charge of +2 (Ca^2+) moved into a neuron and no other ions were moving, it would cause a change in the neuron’s membrane potential. Calcium ions are important for various cellular functions, including neurotransmitter release and muscle contraction. Explanation:
SOLUTION: Naming and Writing Compounds Study Notes – Studypool
Figure 3.3. 1: (a) A sodium atom (Na) has equal numbers of protons and electrons (11) and is uncharged. (b) A sodium cation (Na+) has lost an electron, so it has one more proton (11) than electrons (10), giving it an overall positive charge, signified by a superscripted plus sign. One can use the periodic table to predict whether an atom will Notes on Alginate – Earthworm Express

Source Image: earthwormexpress.com
Download Image
Positive ions Charge Negative ions ammonium NH4+ 1+ chloride Cl− 1− – ppt download Figure 3.3. 1: (a) A sodium atom (Na) has equal numbers of protons and electrons (11) and is uncharged. (b) A sodium cation (Na+) has lost an electron, so it has one more proton (11) than electrons (10), giving it an overall positive charge, signified by a superscripted plus sign. One can use the periodic table to predict whether an atom will
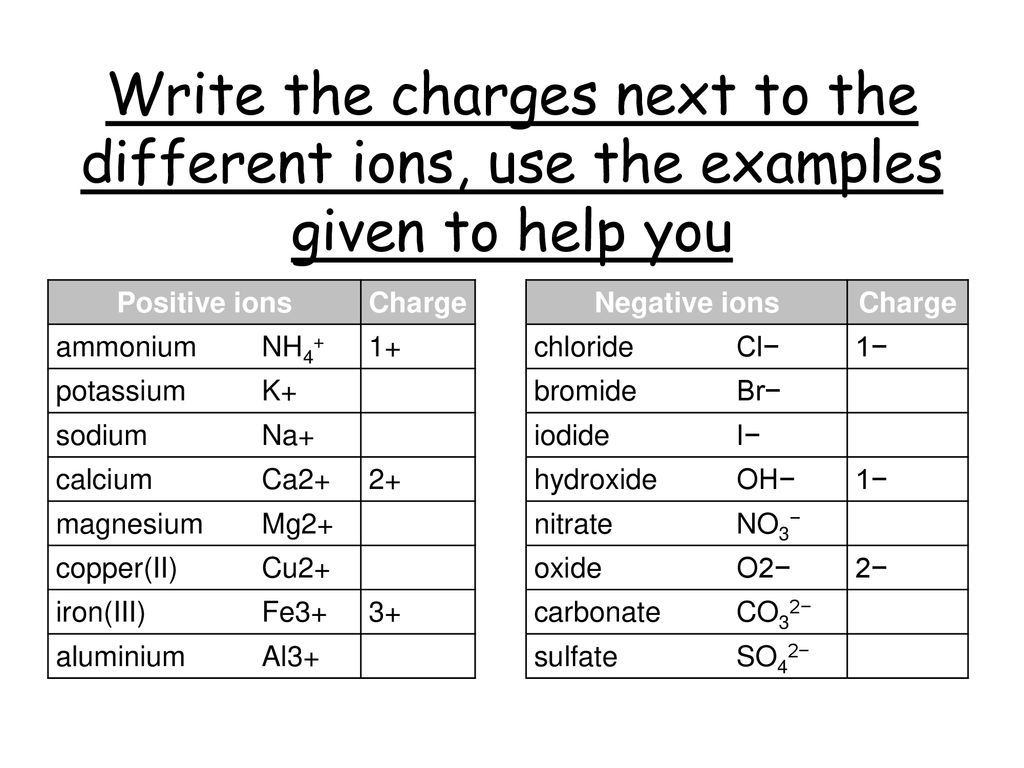
Source Image: slideplayer.com
Download Image
393 Calcium Ions Royalty-Free Photos and Stock Images | Shutterstock The oxygen atom has a 2− charge as an ion. To balance the positive and negative charges, we look to the least common multiple—6: two iron 3+ ions will give 6+, while three 2− oxygen ions will give 6−, thereby balancing the overall positive and negative charges. Thus, the formula for this ionic compound is Fe 2 O 3.
Source Image: shutterstock.com
Download Image
What is TDS in Water & Why Should You Measure It? – Fresh Water Systems The number in the name of the compound close compound A substance formed by the chemical union of two or more elements. shows the charge of the metal ions in that compound. For example, copper(II

Source Image: freshwatersystems.com
Download Image
SOLVED: Calcium has the symbol Ca and its ions have a charge of +2. Chlorine has the symbol Cl and its ions have a charge of -1. In the ionic compound calcium When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most likely to gain one electron to form an ion with a 1- charge. We can use this method to predict the charges of ions in ionic compounds.

Source Image: numerade.com
Download Image
Unveiling the Pros and Cons of Cationic and Anionic Nov 21, 2023Yes. Calcium has a positive 2 charge when it is in its ion form. This would mean that calcium has lost its 2 valence electrons and has 2 more protons than electrons. Calcium Ion

Source Image: microbiozindia.com
Download Image
Battery information guide, all you need to know about batteries Sep 21, 2023bajajsakshi1995 Final answer: If calcium ions with a charge of +2 (Ca^2+) moved into a neuron and no other ions were moving, it would cause a change in the neuron’s membrane potential. Calcium ions are important for various cellular functions, including neurotransmitter release and muscle contraction. Explanation:

Source Image: yuasa.co.uk
Download Image
Positive ions Charge Negative ions ammonium NH4+ 1+ chloride Cl− 1− – ppt download
Battery information guide, all you need to know about batteries Roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron(II) has a 2+ charge; iron(III) a 3+ charge. Anions 1-acetate C 2 H 3 O 2-cyanide CN-amide NH 2-cyanate OCN-hydrogen carbonate fluoride F-(bicarbonate) HCO 3-hydride H-
What is TDS in Water & Why Should You Measure It? – Fresh Water Systems Unveiling the Pros and Cons of Cationic and Anionic When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most likely to gain one electron to form an ion with a 1- charge. We can use this method to predict the charges of ions in ionic compounds.