Video \(\PageIndex1\): A review of precipitation reactions. Chemical reactions are classified according to similar patterns of behavior. A large number of important reactions are included in three categories: precipitation, acid-base, and oxidation-reduction (redox). Precipitation reactions involve the formation of one or more insoluble products.
Delirium: Clinical Manifestation and Management In The Emergency Department | RECAPEM
Classify each of the possible reactions according to whether a precipitate will form or will not form. Precipitate forms: KI(aq) + NaCl(aq) Na2CO3(aq) + CuCl
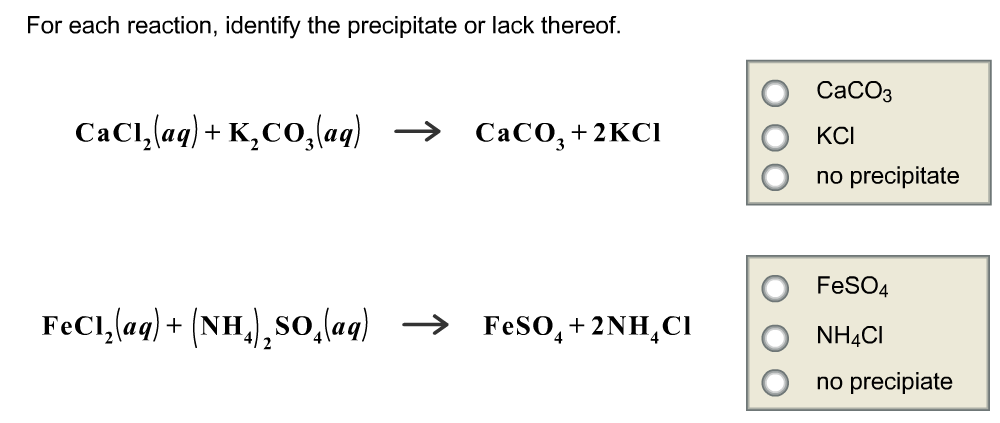
Source Image: ask.learncbse.in
Download Image
Feb 15, 2023The solubility guidelines in Table 4.3.1 4.3. 1 will predict if a precipitation reaction occurs when solutions of soluble ionic compounds are mixed together. To do this, first identify all the ions present in the solution and then consider if any possible cation/anion combination forms an insoluble compound.

Source Image: geeksforgeeks.org
Download Image
Poly(ADP-Ribose) Polymerase Activity and Coronary Artery Disease in Type 2 Diabetes Mellitus | Arteriosclerosis, Thrombosis, and Vascular Biology A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement, double replacement, or metathesis reactions. These reactions are common in nature and are responsible for the formation of coral reefs
Source Image: geologylearn.blogspot.com
Download Image
Classify Each Reaction According To Whether A Precipitate Forms
A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement, double replacement, or metathesis reactions. These reactions are common in nature and are responsible for the formation of coral reefs So let us figure out whether these salts are soluble in water or not. As the rule 1 1 1 states, nitrates are soluble in water, therefore, N a N O 3 \mathbfNaNO_3 NaN O 3 is soluble and will not form a precipitate. Rule 4 4 4 states that sulfates are soluble in water. Therefore, the precipitate will not form.
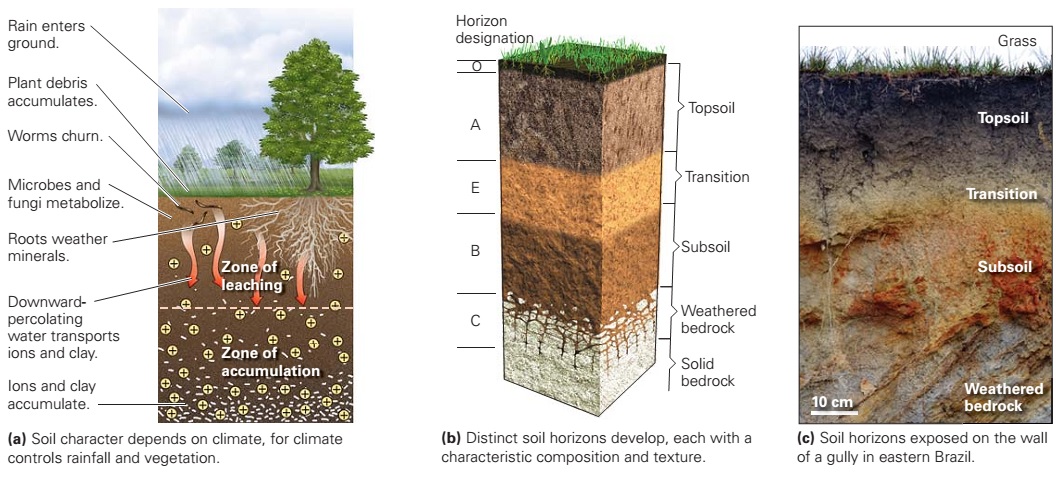
Learning Geology: Soil
Chemistry. Chemistry questions and answers. Question 18 of 9 Classify each of the possible reactions according to whether a precipitate will form or will not form. No reaction Precipitate forms D Answer Bank CrCl (aq) + Li,CO, (aq) .? Kl (aq) + Pb (NO3)2 (aq) – ? Question Video: Determining Which Reaction Will Produce a Precipitate | Nagwa

Source Image: nagwa.com
Download Image
Decomposition reactions require energy either in the form of heat or ligh.. Chemistry. Chemistry questions and answers. Question 18 of 9 Classify each of the possible reactions according to whether a precipitate will form or will not form. No reaction Precipitate forms D Answer Bank CrCl (aq) + Li,CO, (aq) .? Kl (aq) + Pb (NO3)2 (aq) – ?
Source Image: askfilo.com
Download Image
Delirium: Clinical Manifestation and Management In The Emergency Department | RECAPEM Video \(\PageIndex1\): A review of precipitation reactions. Chemical reactions are classified according to similar patterns of behavior. A large number of important reactions are included in three categories: precipitation, acid-base, and oxidation-reduction (redox). Precipitation reactions involve the formation of one or more insoluble products.
Source Image: recapem.com
Download Image
Poly(ADP-Ribose) Polymerase Activity and Coronary Artery Disease in Type 2 Diabetes Mellitus | Arteriosclerosis, Thrombosis, and Vascular Biology Feb 15, 2023The solubility guidelines in Table 4.3.1 4.3. 1 will predict if a precipitation reaction occurs when solutions of soluble ionic compounds are mixed together. To do this, first identify all the ions present in the solution and then consider if any possible cation/anion combination forms an insoluble compound.

Source Image: ahajournals.org
Download Image
Intake of Garlic and Its Bioactive Components – The Journal of Nutrition Define three common types of chemical reactions (precipitation, acid-base, and oxidation-reduction) Classify chemical reactions as one of these three types given appropriate descriptions or chemical equations. Identify common acids and bases. Predict the solubility of common inorganic compounds by using solubility rules.

Source Image: jn.nutrition.org
Download Image
Tumor Necrosis Factor α Receptor Type 1 Activation in the Hypothalamic Paraventricular Nucleus Contributes to Glutamate Signaling and Angiotensin II-Dependent Hypertension | Journal of Neuroscience A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement, double replacement, or metathesis reactions. These reactions are common in nature and are responsible for the formation of coral reefs

Source Image: jneurosci.org
Download Image
2.1 Check-in: Precipitation reactions – Stile So let us figure out whether these salts are soluble in water or not. As the rule 1 1 1 states, nitrates are soluble in water, therefore, N a N O 3 \mathbfNaNO_3 NaN O 3 is soluble and will not form a precipitate. Rule 4 4 4 states that sulfates are soluble in water. Therefore, the precipitate will not form.
Source Image: stileapp.com
Download Image
Decomposition reactions require energy either in the form of heat or ligh..
2.1 Check-in: Precipitation reactions – Stile Classify each of the possible reactions according to whether a precipitate will form or will not form. Precipitate forms: KI(aq) + NaCl(aq) Na2CO3(aq) + CuCl
Poly(ADP-Ribose) Polymerase Activity and Coronary Artery Disease in Type 2 Diabetes Mellitus | Arteriosclerosis, Thrombosis, and Vascular Biology Tumor Necrosis Factor α Receptor Type 1 Activation in the Hypothalamic Paraventricular Nucleus Contributes to Glutamate Signaling and Angiotensin II-Dependent Hypertension | Journal of Neuroscience Define three common types of chemical reactions (precipitation, acid-base, and oxidation-reduction) Classify chemical reactions as one of these three types given appropriate descriptions or chemical equations. Identify common acids and bases. Predict the solubility of common inorganic compounds by using solubility rules.